Step 2: Create Your Store Specific Plan

Resources for Your Plan
As you work through your self-assessment recommendations, you’ll need to access additional resources to help you complete your unique plan. Below are some resources to help you. Please note this may not be the complete list of the tools and resources your store will need.- Bula Intelligence Policy & Procedure Template Section 5.35 a/b/c – Part of a plan requires written standard operating procedures. Download this template to start developing your official policies and procedures manual and customize it according to your pharmacy procedures. There are 3 policy templates to choose from depending on your practice:
+ No-Compounding
+ Non-Sterile Compounding
+ Sterile Compounding
For quick reference, you can download Section 5.35 a/b/c in the Related Documents section on the right sidebar of this page.
Please remember to check the Bula Intelligence tool directly for future updates to the Policies & Procedures template.
- Drug Risk Assessment Form – Not all hazardous drugs have the same level of risk. A risk assessment may be performed to determine alternative containment strategies. NCPA provides templates for you to get started. Click HERE to download the template. Please note there are many different types of drug risk assessment forms that you can locate via a Google search.
- Product Safety Data Sheets – SDS, formerly known as MSDS, are needed for every drug entity your pharmacy stocks. SDS provide information regarding handling, cleaning and disposal. Print and save in a readily accessible area. SDS can be obtained from Drugbank, McKesson Connect or from the manufacture’s website. To access from Connect, click HERE and click on the Safety Data Sheets link under Drug Safety Information.
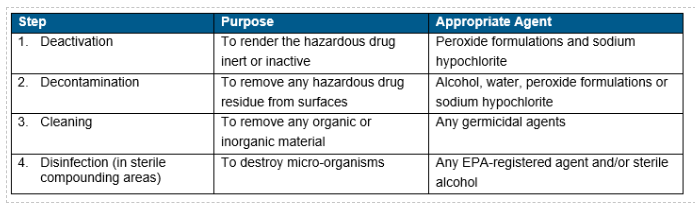
- Deactivating, Decontaminating, Cleaning and Disinfection are essential steps in handling hazardous drugs. Each step serves a unique purpose and may need a specific agent. Refer to SDS for guidelines.
- Hazardous Waste Disposal - Federal, state and local regulations must be followed when disposing all hazardous waste, including unused product, trace contaminated PPE and other materials. Hazardous waste should be separated from non-hazardous waste. Some states require a specific vendor to dispose of hazardous waste. Please reference your state's rules and regulations regarding waste disposal.
Products You May Need
- Protective Personal Equipment (PPE) - Appropriate PPE must be worn when handling hazardous drugs based on your risk assessment and SOPs. Click HERE for a list of PPE products which are available for purchase through McKesson Connect.
- Counting trays – Dedicated equipment is necessary for handling hazardous drugs. Stock up on additional counting trays to minimize workflow disruption.
+ To stock up on counting trays, go to McKesson Connect and search “counting.”
In Conclusion
The purpose of Chapter 800 is to help set quality standards for handling hazardous drugs to promote patient safety, worker safety and environmental protection. At minimum, an entity’s plan must include:
- A list of Hazardous Drugs
- Facility and engineering controls
- Competent personnel
- Safe work practices
- Proper use of appropriate Personal Protective Equipment (PPE)
- Policies for HD waste segregation and disposal
Please download and review the entire USP General Chapter for full details.